“One step to excellence”
Hanmi, your best CDMO partner.
Strength
-
Tailored end-to-end services
-
State-of-the-art cGMP
approved facilities -
Experts for every step
affiliate, Hanmi Fine Chemical, to the development and production of chemically synthesized pharmaceuticals and biopharmaceuticals. Partnership with Hanmi Fine Chemical, Paltan Smart Plant, and
Pyeongtaek Bioplant means the highest quality is assured.
Hanmi Pharmaceutical provides the best CDMO service to customers worldwide based on its R&D capabilities and state-of-the-art facilities. Hanmi aims to be the best partner in realizing customer-tailored services through skilled professionals and top-notch facilities. We want to share our experience in
developing and producing products in the fastest and highest quality manner through optimized routes with healthcare companies around the world.
Bio
VideoHanmi Pyeongtaek Bioplant, located in Pyeongtaek, Gyeonggi-do, Korea, manufactures and supplies excellent biopharmaceutical products
worldwide. Through approvals from MFDS and the US FDA, Pyeongtaek Bioplant is supplying commercial biopharmaceutical products to both the US and Korean markets.
Pyeongtaek Bioplant is equipped with state-of-the-art large-scale manufacturing facilities optimized for the commercial production of microbial-based biopharmaceuticals, including bioreactors with a maximum capacity of 12,500 liters. Additionally, the site houses advanced manufacturing facilities for prefilled syringes, capable of producing over 30 million units annually.
Technology
-
Development to production of
raw materials and finished pharmaceutical products, and comprehensive services for the entire process -
FDA-approved quality systems and manufacturing and management
-
Excellent human resources and state-of-the-art equipment resources, securing flexibility
GMP Testing and Analysis management
- GMP Raw material testing and management
- IPC & Release testing
- Stability testing & Characterization
- Electronic management systems
Biologics GMP Drug Substance Manufacturing
- Microbial Fermentation Process
- Recovery & Purification-Chromatography Process
- PEGylation and Conjugation Process
- DNA and mRNA synthesis
Biologics GMP Drug Product Manufacturing
- Prefilled syringe Aseptic Fill & Finish
- Automatic Visual Inspection
- Final packaging of drug (Blistering & Cartoning)
Status of Certification
| NO | Date | Country | Contents |
|---|---|---|---|
| 1 | Aug. 2007 | Republic of Korea / MFDS | MFDS Pre-approval of GMP Plant (PIC/S) |
| 2 | Nov. 2008 | Republic of Korea / MFDS | MFDS Pre-approval of BGMP |
| 3 | Jan. 2011 | UK / SGS | Certificate of ISO13485 |
| 4 | Jun. 2015 | Republic of Korea / Korea Foundation of Quality | Certificate of ISO14001 |
| 5 | Dec. 2019 | Republic of Korea / Korea Foundation of Quality | Certificate of ISO45001 |
| 6 | May. 2018 | USA / FDA | Premarket approval application(PMA) for SYNOJOYNT |
| 7 | Nov. 2019 | Republic of Korea / MFDS | MFDS GMP Compliance approval of API&DP |
| 8 | Sep. 2022 | USA / FDA | BLA Approval for Rolvedon |
| 9 | May. 2023 | Republic of Korea / MFDS | Certificate of GMP Compliance of a Biopharmaceuticals Contract Manufacturing Organization |
Fine Chemical
HomepageHanmi Fine Chemical is an affiliate of Hanmi Pharmaceutical located in Siheung, Gyeonggi-do, Korea. Fine Chemical is expanding its scope beyond small molecule APIs and Incrementally Modified Drug development by entering the CDMO business.
In addition to cGMP production facilities, API mass production capacity, and
systematic GMP operation, Hanmi Fine Chemical has secured advanced development technologies in mRNA (cap analog, etc.) materials, PEG, and peptides through proprietary R&D based on its accumulated experience in new drug development. ‘Customized service’ is available for each step, from process development and analysis method development to GMP production. Regulatory documents and audits required by health authorities are also fully available.
Technology
-
IP secured Hanmi Cap, PEG, peptide, and pre-established manufacturing facility
-
Inspection experiences by major health authorities and numerous customer audit histories (US FDA, EMA, Japan PMDA, Portugal INFARMED, Australia TGA, etc)
-
Partnership histories with various global
big pharmas (Lilly, Roche, Sanofi, MSD, Janssen, etc)
Reaction
- Glass Lining 75m3(8 sets)
- STS 316L 80m3 (8 sets)
- Hastelloy C-22 40m3 (5 sets), 1m3, 0.5m3, 0.3 m3
- Glass Filter Reactor : 2.5L, 10L, 200L
- Glass Reactor : 100L(2 sets)
Filtration & Drying
- Nutsche Filter 10.2m3(17 sets)
- Centrifuge (basket nominal volume 336L)
- Filter Dryer 15m3(5 sets), Double Cone Vacuum Dryer 3m3, Conical Vacuum Dryer 1.5m3
Lyophilization
- Lyophilizer 20kg, 200kg, 300kg(Ice Capacity)
Milling
- Air Jet mill, Hammer type mill
Status of Certification
| NO | Date | Country | Contents |
|---|---|---|---|
| 1 | May. 2016 | System Korea Certification, Korea Occupational Safety & Health Agency | Renewal of the Safety and Health Management System (ISO 45001, KOSHA-MS) |
| 2 | Jun. 2016 | System Korea Certification | The Quality Management System certification (ISO 14001) reauthentication |
| 3 | Dec. 2019 | System Korea Certification | "The Business Continuity Management System certification (ISO 22301) reauthentication" |
| 4 | Mar. 2023 | Minisry of Employment and Labor | PMS(Process Safety Management) S grade |
Chemical
VideoPaltan Plant is Hanmi Pharmaceutical's central production base for chemical drugs located in Paltan, Gyeonggi-do, Korea. Paltan Smart Plant can rapidly produce more than 6 billion tablets of chemical drugs annually.
Based on its state-of-the-art system and advanced production process, Paltan Plant was accredited by GMP certification from the most pharmaceutically advanced countries and is currently exporting chemical-finished drugs worldwide. The whole process is equipped with the latest ICT (Information and Communication Technology) technology and 90% of the manufacturing and production process is automated.
Technology
-
Smart Plant with automation of process from receiving raw materials to shipping
-
Manufacturing of high-quality pharmaceutical products using information and communication technology (ICT)
-
Excellent manpower and systems to maintain continuous partnerships with global partners
Dispensing
- Direct dispensing to IBC(Intermediate Bulk Container)
- IBC to IBC dispensing system using gravity
Granulation
- Closed System
- Vacuum Transferring System
Storage and Transportation
- Automation of IBC washing
- Automatic warehouse for intermediates
- Unmanned transport device (AGF, Automatic Guided Forklift)
Compression/Capsule Filling
- Closed System
Packaging
- Automatic packaging system (primary packaging → warehousing)
Status of Certification
| NO | Date | Country | Contents |
|---|---|---|---|
| 1 | Jan. 1986 | Republic of Korea / MFDS | MFDS Pre-approval of GMP Plant |
| 2 | Nov. 2017 | Japan / PMDA | MFDS Pre-approval of GMP Plant |
| 3 | Jun. 2019 | Russia / MITRF | Certificate of GMP Compliance |
| 4 | Jan. 2020 | Japan / PMDA | Certificate of GMP Compliance |
Quality
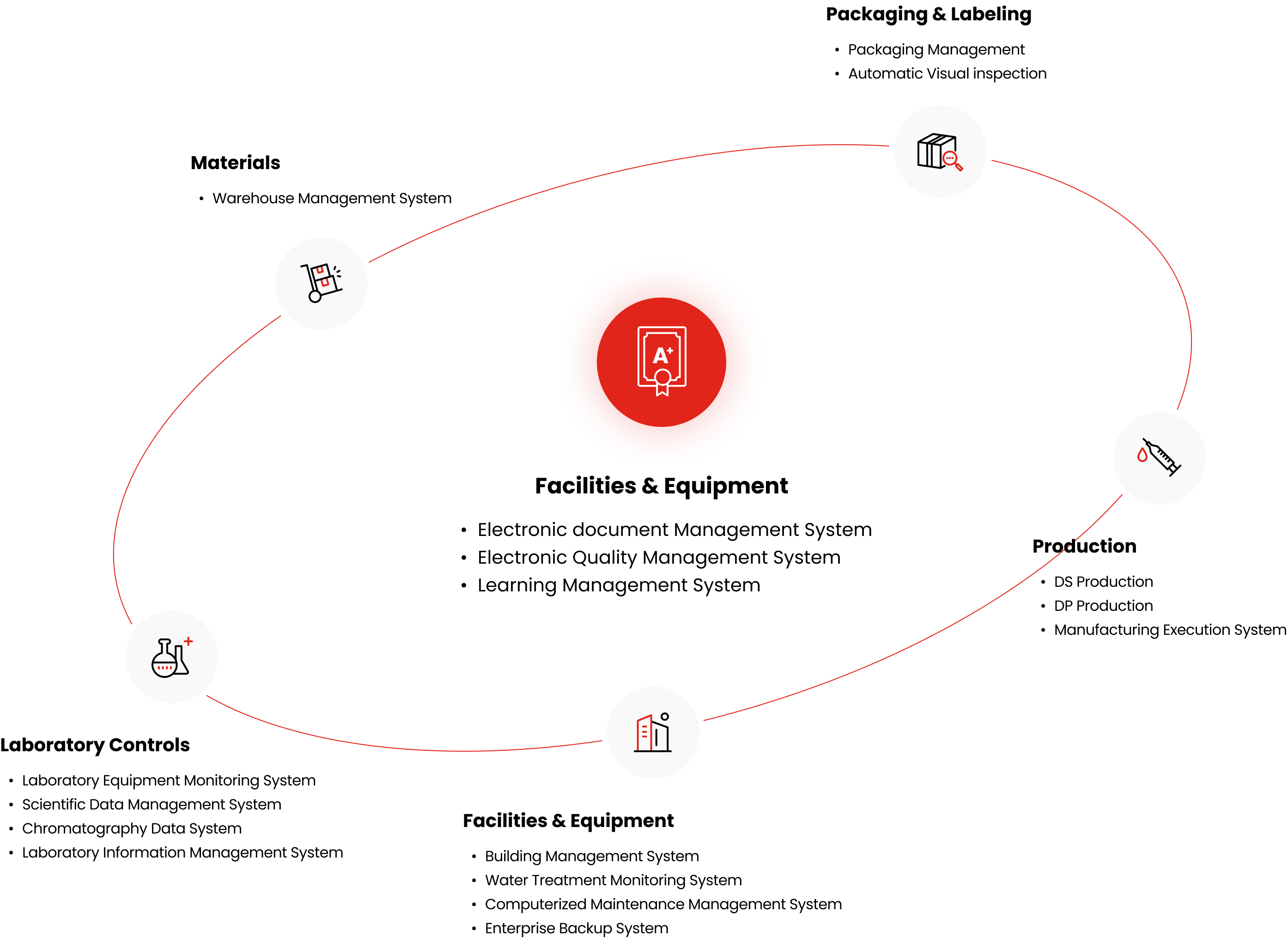
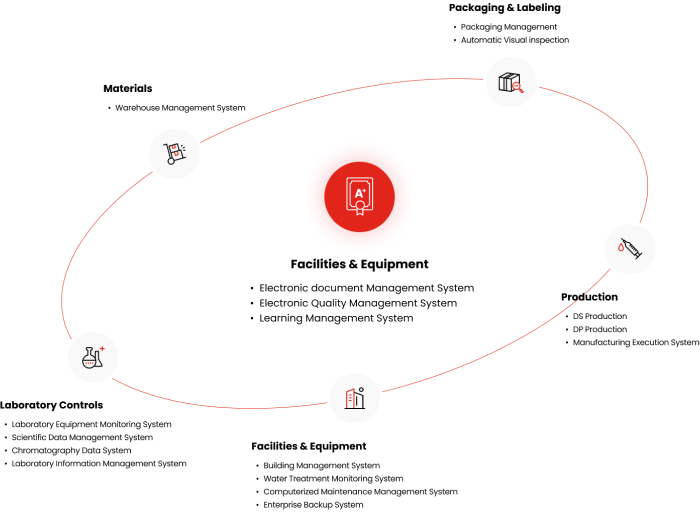
Facilities & Equipment
- Electronic document Management System
- Electronic Quality Management System
- Learning Management System
Packaging & Labeling
- Packaging Management
- Automatic Visual inspection
Production
- DS Production
- DP Production
- Manufacturing Execution System
Facilities & Equipment
- Building Management System
- Water Treatment Monitoring System
- Computerized Maintenance Management System
- Enterprise Backup System
Laboratory Controls
- Laboratory Equipment Monitoring System
- Scientific Data Management System
- Chromatography Data System
- Laboratory Information Management System
Materials
- Warehouse Management System














