Hanmi Files IND with FDA for Novel Obesity Therapy with Muscle-Building Potential
Hanmi Files IND with FDA for Novel Obesity Therapy with Muscle-Building Potential
Targets CRFR2―Not GLP1-R―to Spur Fat Loss and Muscle Gain
Obese Primate Study Demonstrates Selective Fat Reduction Effect
(October 1, 2025) Hanmi Pharmaceutical has taken the first step toward global clinical development of a “next-generation obesity therapy designed to increase muscle mass, following encouraging preclinical and mechanistic study results.”
Hanmi announced on October 1 that it submitted an Investigational New Drug (IND) application to the U.S. Food and Drug Administration (FDA) at the end of last month for HM17321 (LA-UCN2), a novel obesity therapy. The planned Phase 1 clinical trial will evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of HM17321 in healthy adult volunteers.
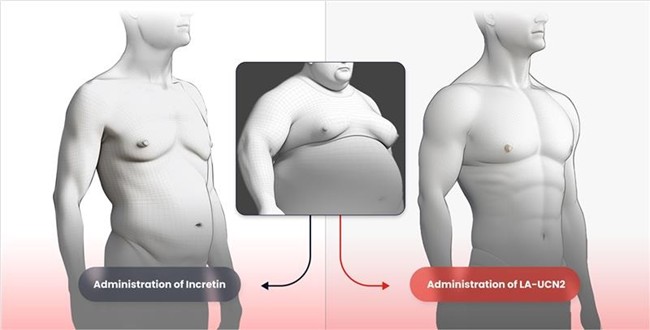
HM17321 is being developed as a first-in-class obesity drug that goes beyond simply muscle- preserving weight loss, achieving the previously unattainable combination of muscle gain and selective fat reduction. Unlike existing GLP-1-based therapies, which inevitably involve muscle loss, HM17321 is poised to become a potential game changer in the global obesity treatment market.
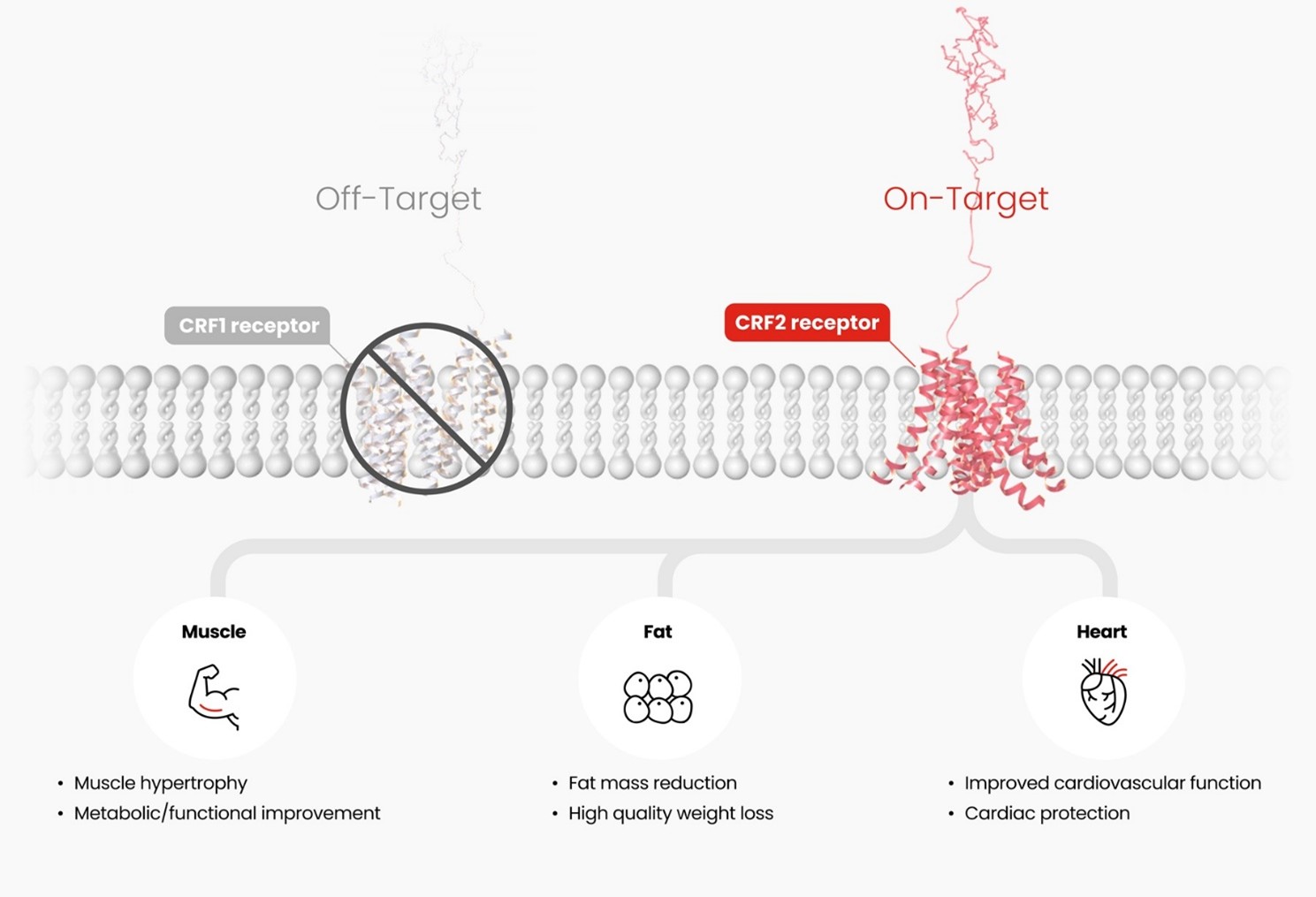
HM17321 is a UCN2 (Urocortin-2) analog selectively targeting the CRFR2 (corticotropin-releasing factor receptor 2) rather than incretin receptors like GLP1-R. It was designed by leveraging Hanmi’s advanced AI and structural modeling platform. CRFR2, a receptor known to be involved in stress recovery, can directly mediate fat reduction, muscle growth, and improvement of muscle function when selectively activated, according to Hanmi.
Since first presenting HM17321’s weight and fat reduction, muscle gain, and functional improvement data at ObesityWeek 2024 last November, Hanmi has steadily strengthened the preclinical evidences through studies presented at ADA 2025 in June using overweight cynomolgus monkeys and at ISMB/ECCB 2025 in August through machine learning-driven animal-to-human translational research, sequentially mitigating uncertainty of this first-in-class drug.
At EASD 2025, Hanmi reported a study further confirming HM17321’s therapeutic efficacy in an obese Rhesus monkey, showing selective fat reduction with preserved lean mass. Another study leveraging proteomics of HM17321 treated muscle elucidated the previously unknown molecular mechanism of CRFR2 agonism causing functional muscle hypertrophy. Series of studies encompassing therapeutic efficacy throughout multiple preclinical species, molecular mechanism, and translational research replenishes HM17321’s potential for clinical success.
HM17321 demonstrates the potential to redefine obesity treatment as a monotherapy, additionally showing quantitatively and qualitatively superior efficacy in weight loss when combined with existing incretin-based therapies, expanding its scope as an innovative treatment.
Most antibody-based muscle-preserving therapies require intravenous administration, limiting convenience for obese patients. Furthermore, combining them with subcutaneously administered obesity therapies presents dosing challenges. More importantly, most of these therapeutics can only maintain muscle mass without improving function, confining themselves in fundamental limitation.
In contrast, HM17321 is peptide molecule, offering convenient administration and competitive cost. As a combination therapy, it can be co-administered with existing incretin-based therapies, which are also peptide drugs, significantly enhancing patient convenience.
Hanmi aims for commercialization by 2031, mobilizing company-wide resources to accelerate clinical development. Following efpeglenatide, targeted for domestic approval in late 2026, and HM15275, the next-generation triple-acting obesity therapy (LA-GLP/GIP/GCG) slated for 2030 commercialization, HM17321 represents the third pipeline in Hanmi’s H.O.P (Hanmi Obesity Pipeline), drawing high expectations.
Hanmi has received FDA approval to initiate a Phase 2 clinical trial with HM15275, which is expected to commence next month, concretizing Hanmi’s next-generation obesity treatment strategy.
In Young Choi, Head of R&D Center, said, “Hanmi’s drug development strategy views obesity treatment not merely as a competition for weight loss but as a journey to help patients achieve sustainable, long-term health. HM17321 targets fat reduction, muscle growth, and functional and metabolic improvement simultaneously, addressing unmet needs in sarcopenic and elderly obese populations, as well as patients with impaired exercise capacity. We are confident it will establish itself as an innovative therapy in the near future.”
Hanmi will present four key studies on HM15275 and HM17321 at ObesityWeek 2025 in Atlanta this November.
■ Contact info:
Hanmi Pharmaceutical (www.hanmipharm.com)
innovation@hanmi.co.kr, +82-02-410-0467